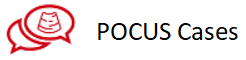An 82-year-old patient underwent elective surgery (right hemicolectomy) due to the adenocarcinoma of the ascending colon.
On the 5th day, the patient vomited intestinal content. She was admitted to the ICU for respiratory insufficiency several hours later. The patient had to be intubated (after short non-invasive ventilation). Shock developed during mechanical ventilation and levels of inflammatory markers increased very significantly. High-dosed noradrenaline was needed 2.5 mg/h.
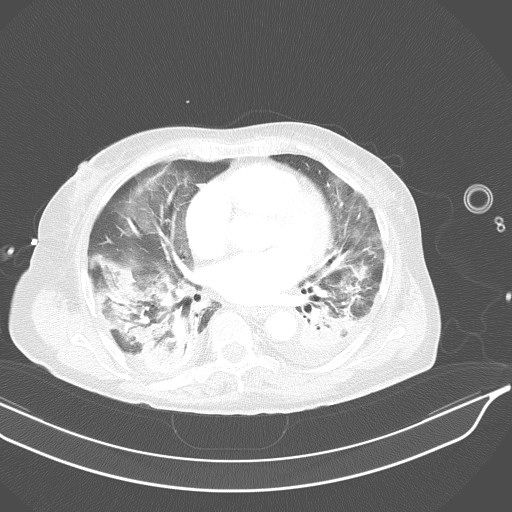
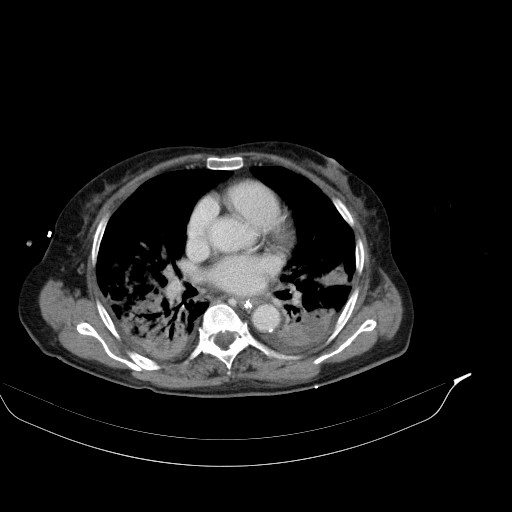
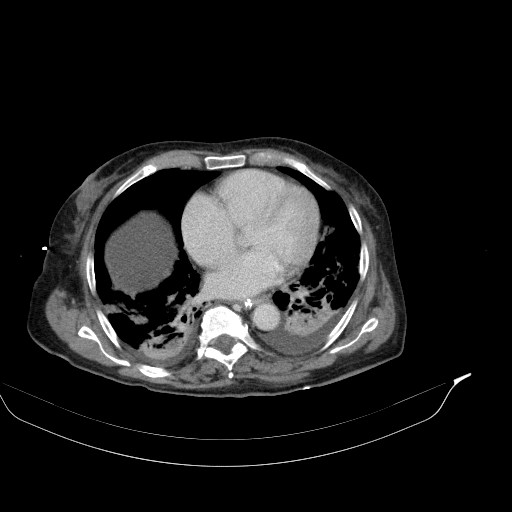
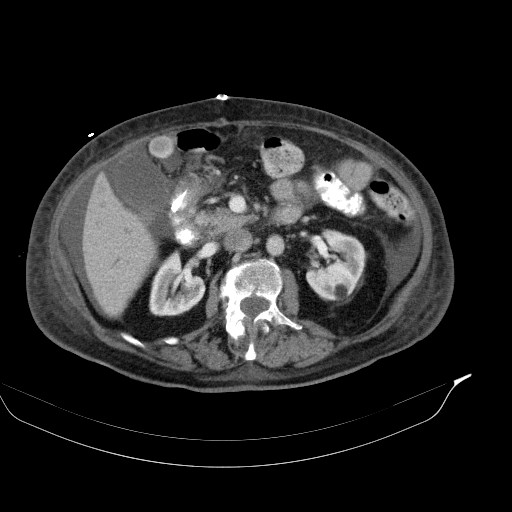
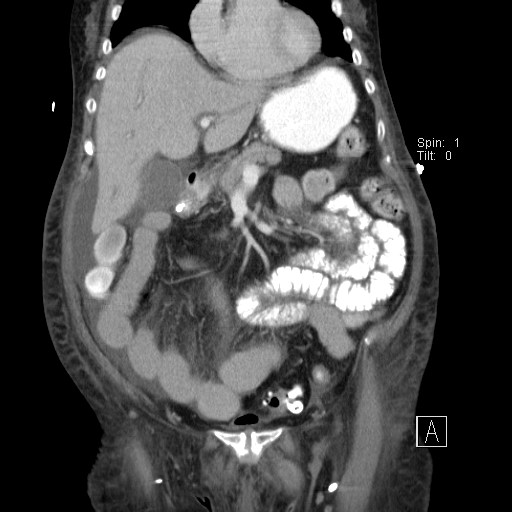
CT of the abdomen and thorax was performed. Inflammatory infiltrations were found in lower lobes of both lungs as well as a small amount of ascites with traces of free air ventrally (after previous surgery?). The small intestine was dilated across its whole length up to the anastomosis, which was probably obturated (suspicion of oedema).
Bilateral aspirational pneumonia was determined as the cause of sepsis.
Ultrasonography was performed to:
- assess the pathophysiology of the shock and to
- determine the focus of infection (already sepsis).
A4C
Interventricular septum and lateral wall of left ventricle both present with good kinetics. Right ventricle shows a good systolic function and no dilation. There is a slight dilation of the left atrium.
A4C/A5C, CFM
Mild mitral regurgitation which is difficult to assess in the terrain of tachycardia. Aortic regurgitation is not detected.
A4C, TDI sample volume is placed above lateral mitral annulus
Measurement of E´-wave. S´-wave is normal, which points out to good global kinetics of the left ventricle.

A4C, CW line is placed across the aortic valve
Maximum of the flow acceleration is around 2 m/s, which excludes any significant aortic stenosis.
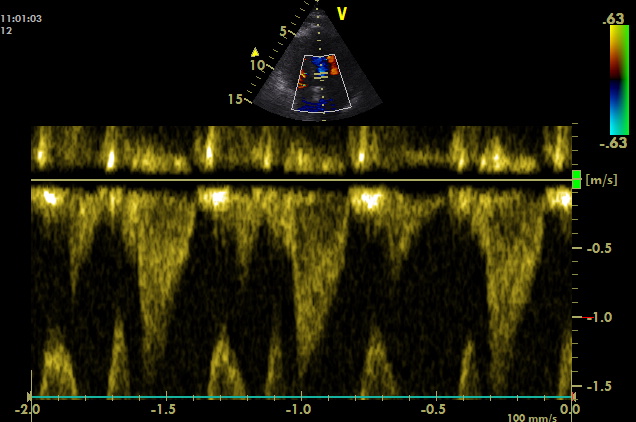
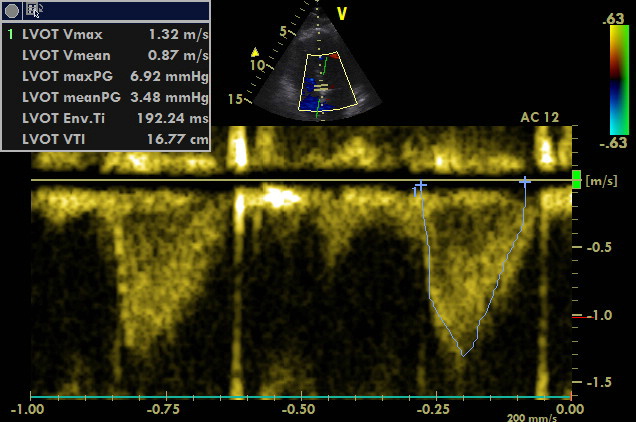
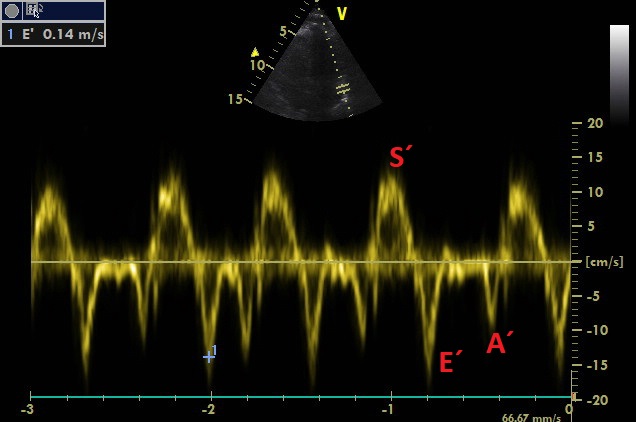
A4C, PW sample volume is placed at LVOT
Maximum of flow acceleration in LVOT is above 1 m/s without signs of dynamic obstruction (based on spectral Doppler measurement). VTI of 16.77 cm corresponds to the stroke volume of approximately 50 ml. Thus, cardiac output is approximately 6 l/min (120 bpm). Cardiac index is above 3 l/min/m2.
A3C, visualisation was possible only during expiration
Anterior septum and inferolateral wall of the left ventricle both present with good kinetics. Mitral valve does not show any signs of pathology. No separation of pericardial space.
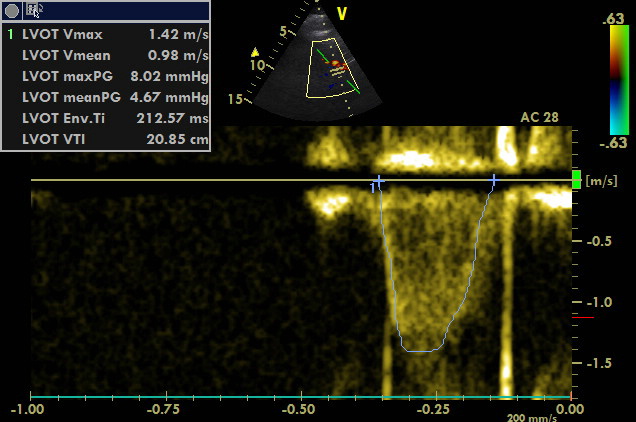
A3C, PW sample volume is placed at LVOT
VTI of 20.85 cm corresponds to the stroke volume of 60 ml. Thus, cardiac output exceeds the value of 7 l/min (120 bpm). Cardiac index is above 3.5 l/min/m2.
Conclusion of echocardiography: Normo-hypokinesia of both ventricles, the cardiac index is estimated to be above 3.5 l/min/m2.
Fluid responsiveness was not assessed considering the absence of signs of peripheral hypoperfusion (capillary refill time below 2 seconds, the physiological value of lactate and preserved diuresis) and respiratory failure with the necessity of high PEEP (12) and FiO2 (60%). We did not change the dose of noradrenaline.
Then, we started to look for the focus of the infection. Was the aspiration pneumonia the cause? Could a postoperative intrabdominal infection be also present?
Lung ultrasonography:
A-profile was found at all BLUE points. The recordings were not saved.
Coronal scanning plane at right PLAPS point
There is fluid (and liver) below the diaphragm. There is a consolidation of the caudal part of inferior lobe of the right lung. It shows shred sign. Significant pleural effusion is not detected.
Coronal scanning plane at left PLAPS point
Below diaphragm, there is the dilated stomach with fluid inside. The spleen becomes visible when the scanning plane is tilted dorsally. Ascites is not detected. Irregular lung line appears above diaphragm during inspiration. Shred sign is present.
Conclusion of lung ultrasonography: The finding corresponds to bilateral dorsobasal consolidations caused by inflammatory infiltration (pneumonia).
The patient received antibiotics. Her condition improved during the two following days. It was possible to go down to the pressure support ventilation (CPAP/ASB) with a low FiO2 and PEPP with simultaneous stabilisation of haemodynamics.
On the 3rd day, the respiratory condition worsened again with the necessity of high FiO2 (60%) and increasing of PEEP (maximum of 10 cm H2O, the further increase was not tolerated haemodynamically). The sedation had to be deepened, and we went back to the mandatory mechanical ventilation.
Check ultrasonography:
Sagittal scanning plane at right upper BLUE point
Many B-lines are originating from the pleural line in the cranial intercostal space. There is also a subpleural consolidation with sub-B-line. In the caudal intercostal space (on the right side of recording) there is A-profile. C-profile.
Sagittal scanning plane at right lower BLUE point
An image of merging B-lines (Birroleau type) alternates with A-profile (A-line is visible). Focal interstitial syndrome.
Sagittal scanning plane at left lower BLUE point
An image of merging B-lines (Birroleau type) alternates with A-profile (trace of A-line). Focal interstitial syndrome.
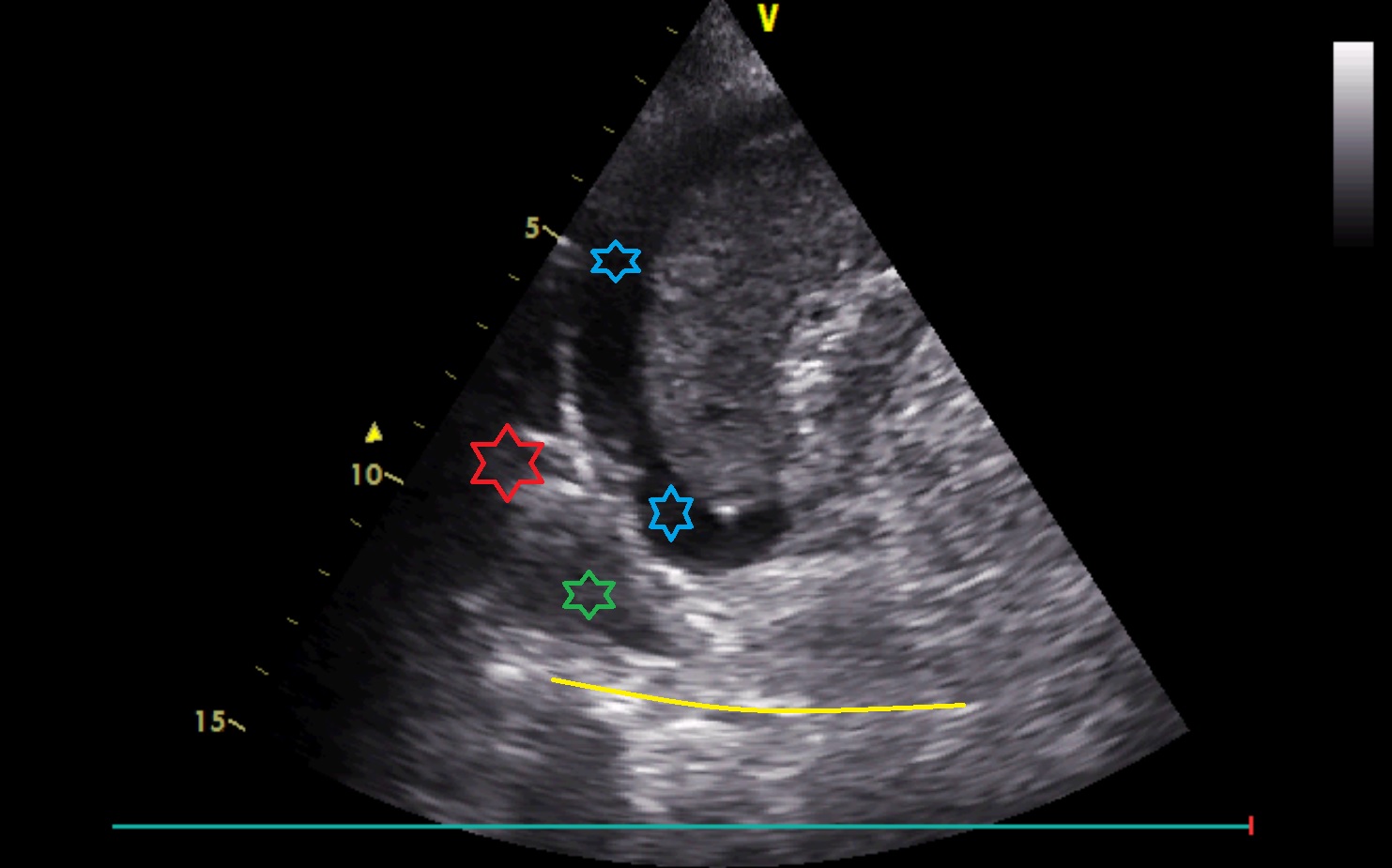
Coronal scanning plane caudally to left PLAPS point
There is ascites around the spleen (blue colour). Medially above the diaphragm in the cardiophrenic angle, there is an irregular hyperechoic structure (corresponding to well-aerated lung, red colour) and small pleural effusion (green colour) enabling visualisation of the vertebral column above the diaphragm (V-sign, yellow colour).
Subcostal oblique scanning plane at the right mid-clavicular line
Below inferior margin of the liver, there is the gallbladder. Its wall is thickened. There is sludge in its lumen. Above sludge, there are hyperechoic structures ventrocaudally without acoustic shadows below them. Ascites below liver becomes visible when the scanning plane is tilted laterally.
The probe was rotated by 90°:
Subcostal oblique scanning plane at the right mid-clavicular line
Below inferior margin of the liver, there is the gallbladder. Its wall is thickened. There is sludge in its lumen. Above sludge, there are hyperechoic structures ventrocaudally without acoustic shadows below them.
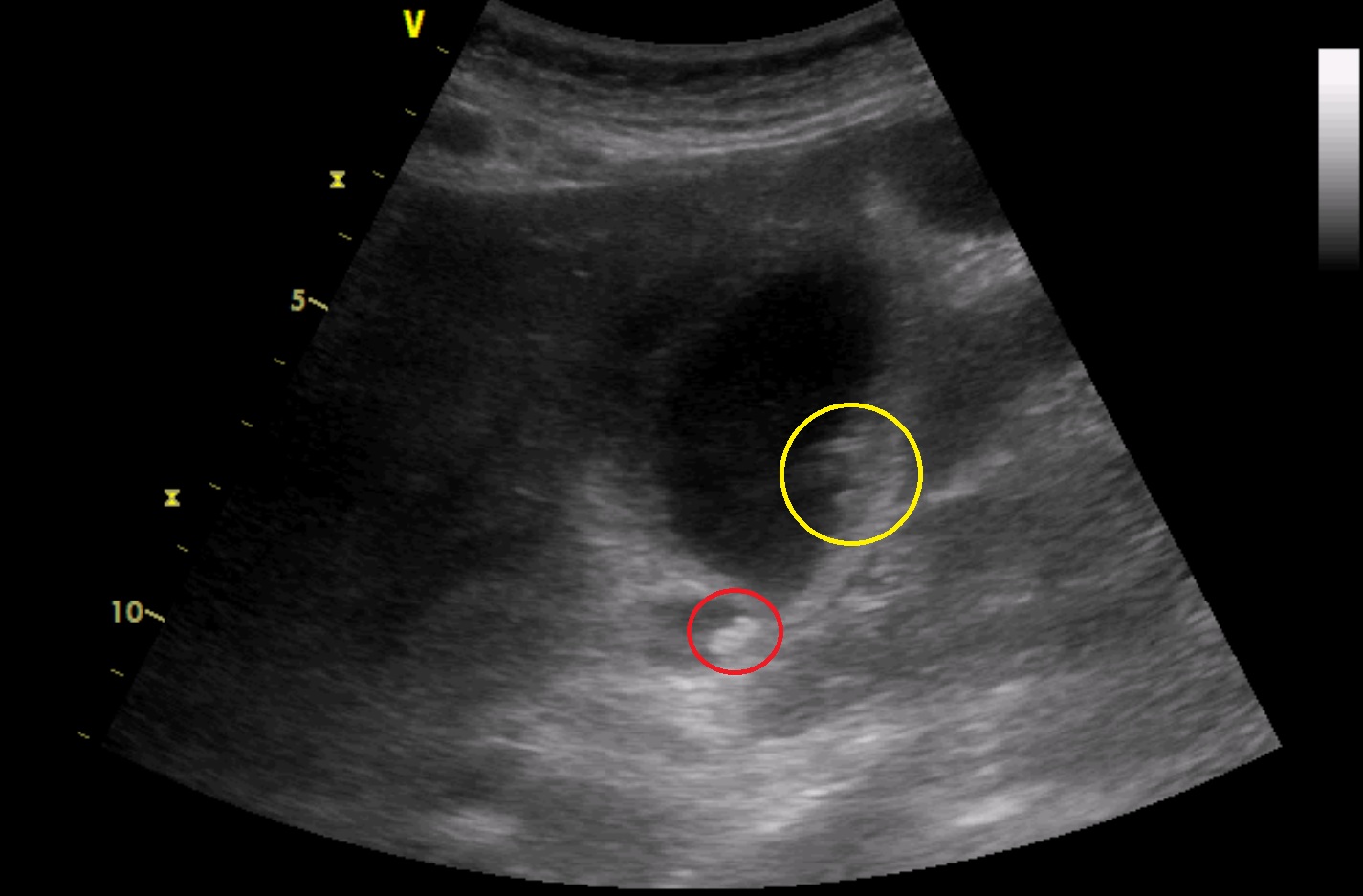
Subcostal oblique scanning plane at the right mid-clavicular line
Below inferior margin of the liver, there is the gallbladder. Its wall is thickened. There is sludge in its lumen. Above sludge, there are hyperechoic structures ventrocaudally without acoustic shadows below them (yellow colour). In the region of gallbladder neck, there is another hyperechoic structure without an acoustic shadow below it (red colour). There is a small amount of fluid caudally to the gallbladder.
Focus on the hyperechoic structure in the region of gallbladder neck:

Subcostal oblique scanning plane at the right mid-clavicular line
In the area of the gallbladder neck, there is another hyperechoic structure. Detailed examination reveals a weak acoustic shadow below it (red colour).
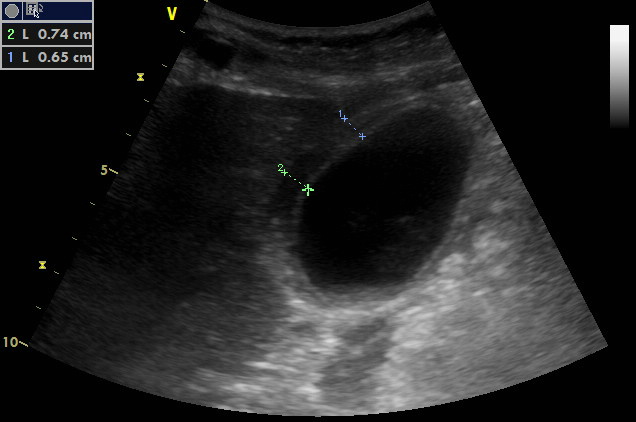
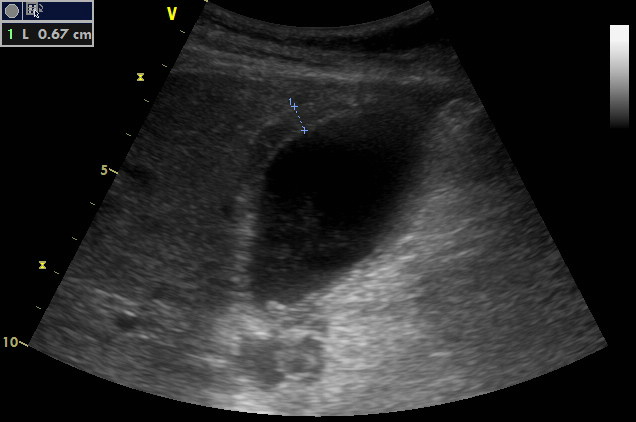
Subcostal oblique scanning plane at the right mid-clavicular line
Measurement of the gallbladder wall thickness. It seems to have three layers and is definitely thickened.
Visualisation of the hepatic hilum:
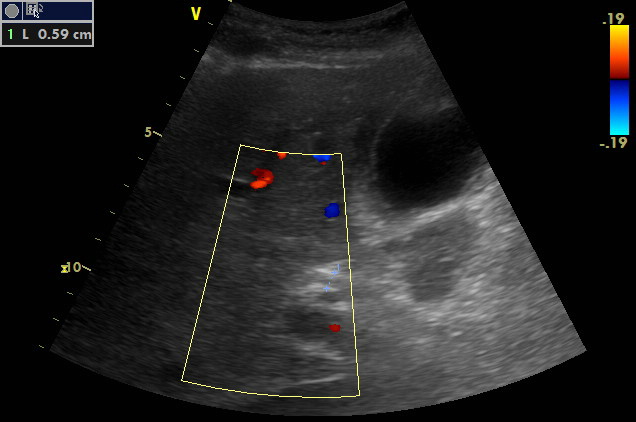
Subcostal oblique scanning plane at the right anterior axillary line, CFM
Portal triad is visualised. There is the portal vein dorsally. Ventrally to it, there is the proper hepatic artery (art.hepatica propria). It shows a tortuous track. It may be doubled or already branched. Hepatic duct (DHC) is not dilated (5.9 mm).
Conclusion of lung and abdominal ultrasonography:
- Pleural effusion on the left side.
- Bilateral inflammatory infiltrations of both lungs basally without a significant translobar consolidation bilaterally.
- Suspicion of the ARDS – diffuse lesions of the focal interstitial syndrome and small subpleural consolidations alternate with physiological A-profile without clear boundaries between them (so-called spared areas) ventrally and bilaterally.
- Calculous cholecystitis may be present.
Next steps:
Thickening of the gallbladder wall is less specific for the diagnosis of acute cholecystitis in critically ill patients than in healthy people. It was not possible to examine sonographic Murphy sign by applying pressure to the probe. The finding was consulted with a surgeon. Ascites was punctured and was serous macroscopically. Thus, surgical intervention was not indicated.
An attempt to titrate PEEP and change to mechanical ventilation was followed by immediate significant hypotension and bradycardia. The dose of noradrenaline was increased. A bolus of a small dose of adrenalin was administered. Haemodynamics stabilised at the level of 2.5 mg/h of noradrenalin. Maximum PEEP of 8 cm H2O was hemodynamically tolerated.
Check echocardiography:
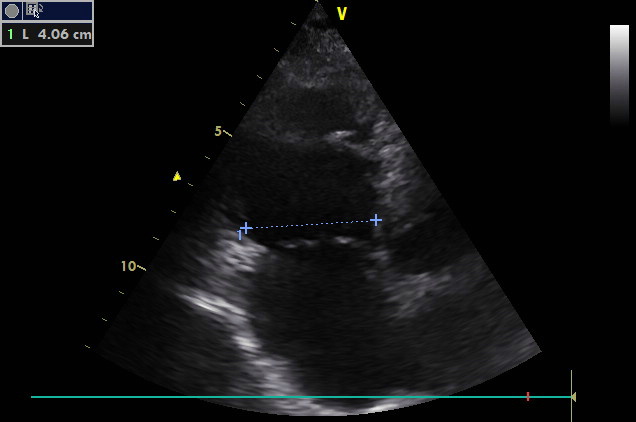
A4C, focus on the right side of the heart
Borderline size and global right ventricular systolic dysfunction. The tricuspid valve is smooth. Dilation of the right atrium.
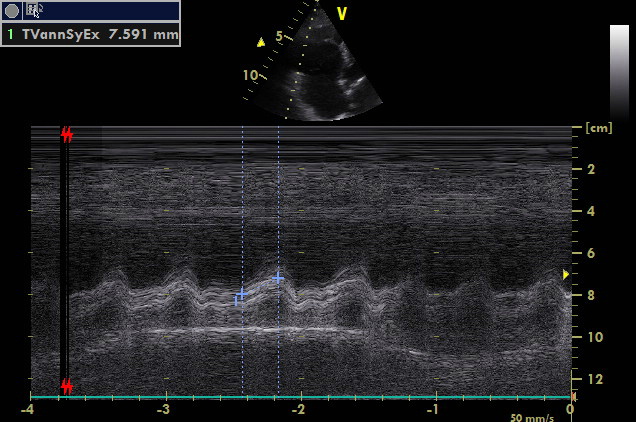
A4C, focus on the right side of heart, M mode
Very low TAPSE points out to significant global right ventricular systolic dysfunction.

A4C, CFM
Significant tricuspid regurgitation is visualised. Its gradient is measured. A significant elevation of pulmonary artery pressure is not detected.
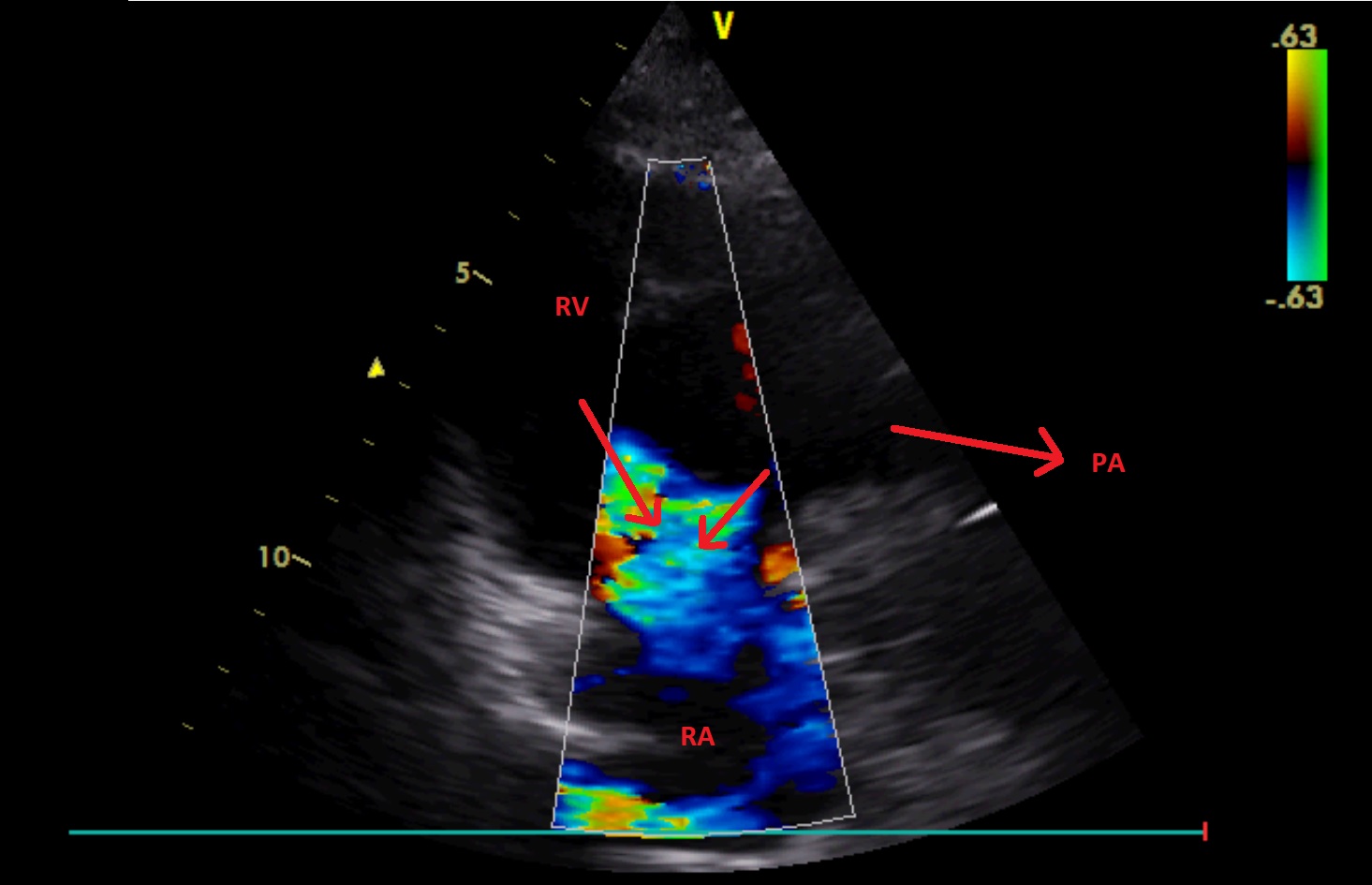
A2C – right ventricle (focus on the right side of the heart), CFM
Significant tricuspid regurgitation is visualised. It has two jets.
A4C, an attempt to visualise the left side of the heart
Left ventricular systolic dysfunction. Its lateral wall presents with hypokinesia; however, the quality of the imaging is not sufficient.
Subcostal scanning plane, inferior vena cava is visualised at its longitudinal axis
Inferior vena cava is dilated. It does not show any variability in the terrain of mechanical ventilation (the cursor is placed cranially).
Conclusion of echocardiography:
- Acute right ventricular dysfunction without elevation of pulmonary artery pressure – differential diagnosis encompasses infarction of right ventricle and septic cardiomyopathy with right ventricular failure due to elevation of its afterload in the terrain of ARDS. Acute right ventricular failure in the ground of septic cardiomyopathy was more probable considering haemodynamic decompensation directly related to the change to mechanical ventilation and elevation of PEEP. Compare the actual function of the right ventricle and its function at the beginning of the case.
- Left ventricular dysfunction, it is also caused by septic cardiomyopathy.
Next steps:
The patient was positioned to the prone position. The patient tolerated increasing of PEEP in this position. Oxygenation improved. The dose of noradrenalin could be reduced by more than 25%. Continuous renal replacement therapy with the goal of negative fluid balance was started.
What happened next:
ARDS withdrew thanks to antibiotic therapy; the intestinal passage was restored without the necessity of surgical intervention.
Unfortunately, the patient´s therapy was changed to palliative care due to critical illness polyneuropathy and persisting renal failure. Later on, she died.
CT findings for comparison: